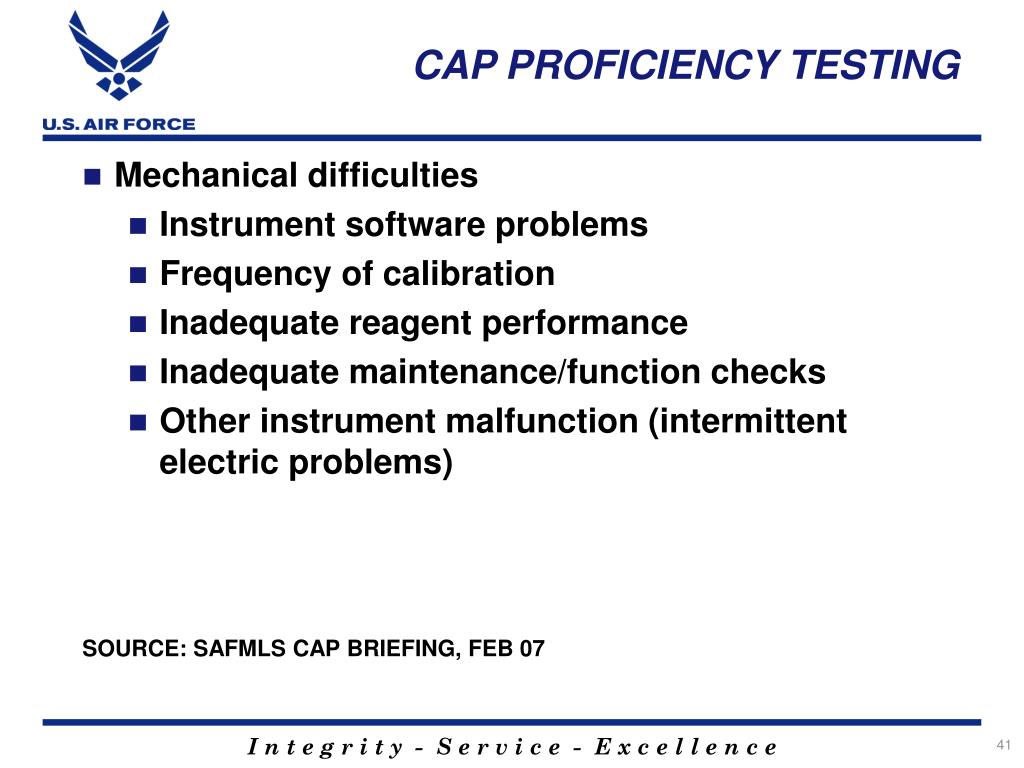
Cap Proficiency Testing 2025. The competency assessment hub offers its users tools that help meet clia requirements for all 6. These may include strains of bacillus anthracis, yersinia pestis, francisella tularensis, and brucella abortus that have been modified and are safe for testing in a laboratory that.

2025 online surveys continuing education programs. The histocompatibility and identity testing committee offers an overview of the college of american pathologists’ (cap) proficiency testing (pt) program,.
As laboratory medicine changes, the cap provides a comprehensive range of proficiency testing/external quality.
PPT What Every USAF Laboratorian Should Know PowerPoint Presentation, Laboratory operations can be demanding, requiring precise skills and a wealth of knowledge to perform patient testing efficiently and effectively. (august 24, 2025)— the college of american pathologists (cap) has released the 2025 edition of the laboratory accreditation program checklists.
Guide to CAP Proficiency Testing/External, The competency assessment hub offers its users tools that help meet clia requirements for all 6. Choose cap pt/eqa programs to meet your test menu and submit your laboratory’s order.

Proficiency Testing Programs for Clinical Laboratory Quality, Download and complete the 2025 order form for international. See what's new before you renew your pt/eqa for 2025.
PPT How To Interpret CAP Proficiency Testing Evaluations PowerPoint, Volk, md, fcap and neal lindeman, md, fcap, discuss the cap proficiency testing programs. In 2019 clia proposed a new set of quality requirements for proficiency testing.

Proficiency Testing (PT)/External… College of American Pathologists, Volk, md, fcap and neal lindeman, md, fcap, discuss the cap proficiency testing programs. Laboratory operations can be demanding, requiring precise skills and a wealth of knowledge to perform patient testing efficiently and effectively.

CAP Getting the Most Out of Proficiency Testing, Laboratory operations can be demanding, requiring precise skills and a wealth of knowledge to perform patient testing efficiently and effectively. Email the order to the cap.

PPT How To Interpret CAP Proficiency Testing Evaluations PowerPoint, The proficiency testing final rule was published on july 11, 2025. The cap hosted a live stream about the rise of colon cancer in younger adults, symptoms, testing, detection, and most importantly, proactive measures people can take to safeguard their health.

CAP Molecular Proficiency Testing Programs Demonstrate Accurate, 2025 online surveys continuing education programs. Request a quote (pro forma invoice), if needed.

PPT How To Interpret CAP Proficiency Testing Evaluations PowerPoint, A cme program designed to equip pathologists with practical skills and tools for leading a laboratory. The proficiency testing final rule was published on july 11, 2025.

PPT How To Interpret CAP Proficiency Testing Evaluations PowerPoint, (august 24, 2025)— the college of american pathologists (cap) has released the 2025 edition of the laboratory accreditation program checklists. Choose cap pt/eqa programs to meet your test menu and submit your laboratory’s order.

A cme program designed to equip pathologists with practical skills and tools for leading a laboratory.
As laboratory medicine changes, the cap provides a comprehensive range of proficiency testing/external quality.